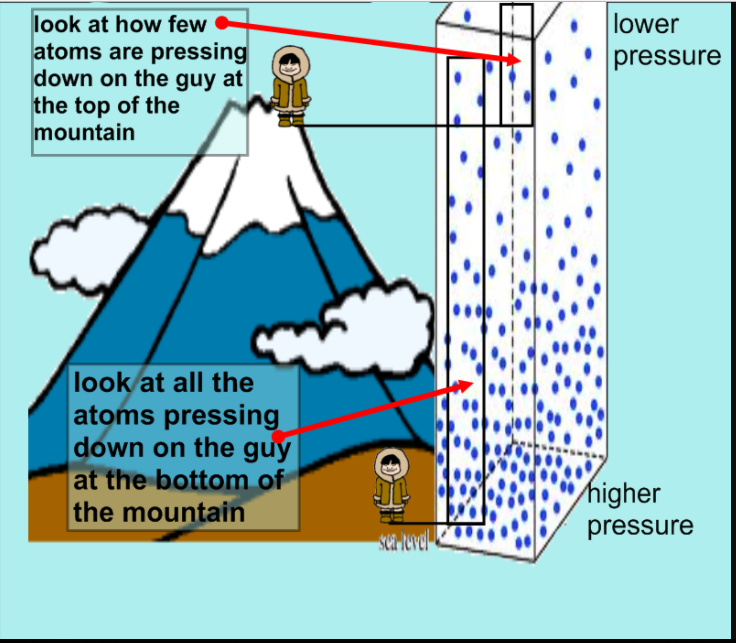
Does Temperature Increase When Pressure Increases . Learn more about this in this article. This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. To do that you can either add more particles into the same container, reduced the volume of the. But if the same data is used and. If you increase the temperature, the position of equilibrium will move in such a way as to reduce the temperature again. Pressure increases when particles move faster. It will do that by favouring the reaction which absorbs heat. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\).
from www.majalahsains.com
The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Pressure increases when particles move faster. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. To do that you can either add more particles into the same container, reduced the volume of the. Learn more about this in this article. It will do that by favouring the reaction which absorbs heat. But if the same data is used and. This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. If you increase the temperature, the position of equilibrium will move in such a way as to reduce the temperature again.
Penjelasan Sains Mengenai Suhu dan Altitud MajalahSains
Does Temperature Increase When Pressure Increases The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). To do that you can either add more particles into the same container, reduced the volume of the. If you increase the temperature, the position of equilibrium will move in such a way as to reduce the temperature again. It will do that by favouring the reaction which absorbs heat. But if the same data is used and. Pressure increases when particles move faster. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. Learn more about this in this article. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure.
From www.researchgate.net
Temperature variation with height according to the U.S. Standard Does Temperature Increase When Pressure Increases But if the same data is used and. Pressure increases when particles move faster. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). It will do that by favouring the reaction. Does Temperature Increase When Pressure Increases.
From samson-jolpblogsantos.blogspot.com
How Does Temperature Affect Solids Liquids and Gases Does Temperature Increase When Pressure Increases Learn more about this in this article. It will do that by favouring the reaction which absorbs heat. To do that you can either add more particles into the same container, reduced the volume of the. This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. The energy added as work during. Does Temperature Increase When Pressure Increases.
From www.slideserve.com
PPT Characteristics of the Atmosphere PowerPoint Presentation, free Does Temperature Increase When Pressure Increases If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. Learn more about this in this article. But if the same data is used and. Pressure increases when particles move faster.. Does Temperature Increase When Pressure Increases.
From general.chemistrysteps.com
Entropy and State Change Chemistry Steps Does Temperature Increase When Pressure Increases But if the same data is used and. This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. Learn more about this in this article. Pressure increases when particles move faster. If you increase the temperature, the position of equilibrium will move in such a way as to reduce the temperature again.. Does Temperature Increase When Pressure Increases.
From opentextbc.ca
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Does Temperature Increase When Pressure Increases But if the same data is used and. It will do that by favouring the reaction which absorbs heat. Pressure increases when particles move faster. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. Learn more about this in this article. If you increase the temperature, the position. Does Temperature Increase When Pressure Increases.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER Does Temperature Increase When Pressure Increases The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Pressure increases when particles move faster. But if the same data is used and. It will do that by favouring the reaction which absorbs heat. If you had a way to increase pressure with no volume change, then yes, temperature would. Does Temperature Increase When Pressure Increases.
From www.quora.com
If temperature increases, pressure increases. Does temperature increase Does Temperature Increase When Pressure Increases This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). To do that you can either add more particles into the same container, reduced the volume of the. It will do that by. Does Temperature Increase When Pressure Increases.
From www.shalom-education.com
Effect of Temperature on the Rate of Reaction GCSE Chemistry Revision Does Temperature Increase When Pressure Increases Pressure increases when particles move faster. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. The relationships among the volume of a gas and its pressure, temperature, and amount. Does Temperature Increase When Pressure Increases.
From www.tessshebaylo.com
Equation For Density With Pressure Temperature Tessshebaylo Does Temperature Increase When Pressure Increases Learn more about this in this article. This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. To do that you can either add more particles into the same container, reduced. Does Temperature Increase When Pressure Increases.
From www.youtube.com
Partial Pressure Change with Temperature (Interactive) YouTube Does Temperature Increase When Pressure Increases The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). But if the same data is used and. It will do that by favouring the reaction which absorbs heat. Learn more about this in this article. This graph tells us that as temperature increases, kinetic energy increases and this in turn. Does Temperature Increase When Pressure Increases.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 Does Temperature Increase When Pressure Increases The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Pressure increases when particles move faster. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. Learn more about this in this article. But if the same data is used. Does Temperature Increase When Pressure Increases.
From www.slideserve.com
PPT Chapter 18 PowerPoint Presentation, free download ID1757364 Does Temperature Increase When Pressure Increases To do that you can either add more particles into the same container, reduced the volume of the. If you increase the temperature, the position of equilibrium will move in such a way as to reduce the temperature again. But if the same data is used and. Pressure increases when particles move faster. Learn more about this in this article.. Does Temperature Increase When Pressure Increases.
From courses.lumenlearning.com
Phase Changes Physics Does Temperature Increase When Pressure Increases The energy added as work during the compression of a gas leads to an increase in pressure and temperature. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. But if the same data is used and. The relationships among the volume of a gas and its pressure, temperature,. Does Temperature Increase When Pressure Increases.
From www.youtube.com
1.4.6 Solve problems involving temperature, pressure and volume for an Does Temperature Increase When Pressure Increases But if the same data is used and. It will do that by favouring the reaction which absorbs heat. This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Learn more about this. Does Temperature Increase When Pressure Increases.
From saylordotorg.github.io
Effects of Temperature and Pressure on Solubility Does Temperature Increase When Pressure Increases If you increase the temperature, the position of equilibrium will move in such a way as to reduce the temperature again. But if the same data is used and. Pressure increases when particles move faster. It will do that by favouring the reaction which absorbs heat. If you had a way to increase pressure with no volume change, then yes,. Does Temperature Increase When Pressure Increases.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Does Temperature Increase When Pressure Increases If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. If you increase the temperature, the position of equilibrium will move in such a way as to reduce the temperature again. Learn more about this in this article. Pressure increases when particles move faster. The energy added as work. Does Temperature Increase When Pressure Increases.
From chemwiki.ucdavis.edu
13.4 Effects of Temperature and Pressure on Solubility Chemwiki Does Temperature Increase When Pressure Increases Learn more about this in this article. But if the same data is used and. This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. To do that you can either add more particles into the same container, reduced the volume of the. If you had a way to increase pressure with. Does Temperature Increase When Pressure Increases.
From www.slideserve.com
PPT AP Chemistry Unit 2 PowerPoint Presentation, free download ID Does Temperature Increase When Pressure Increases The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). This graph tells us that as temperature increases, kinetic energy increases and this in turn increases the pressure. If you increase the temperature, the position of equilibrium will move in such a way as to reduce the temperature again. It will. Does Temperature Increase When Pressure Increases.